 According to the definition by the World Health Organisation (WHO), probiotics are live, non-pathogenic microorganisms that, when administered in adequate amounts, confer a health benefit on the host. Most probiotics belong to the group of lactic acid bacteria. These concern gram positive, coccoid or rod-shaped, immobile bacteria which, due to their metabolic activity, produce lactic acid. The most important, probiotically relevant representatives of lactic acid bacteria belong to the genus lactobacilli and bifidobacteria. Known types are L. acidophilus, L. casei and L. rhamnosus as well as B. bifidum, B. breve and B. longum.
According to the definition by the World Health Organisation (WHO), probiotics are live, non-pathogenic microorganisms that, when administered in adequate amounts, confer a health benefit on the host. Most probiotics belong to the group of lactic acid bacteria. These concern gram positive, coccoid or rod-shaped, immobile bacteria which, due to their metabolic activity, produce lactic acid. The most important, probiotically relevant representatives of lactic acid bacteria belong to the genus lactobacilli and bifidobacteria. Known types are L. acidophilus, L. casei and L. rhamnosus as well as B. bifidum, B. breve and B. longum.
Based on their composition, there are three different types of probiotic:
- Monostrain probiotics consist of just one particular type or genus of microorganism strain.
- Multistrain probiotics contain more than one strain of the same or a related microorganism.
- Multispecies probiotics consist of strains of various probiotics types which belong to one or more genera.
Functions of probiotics
Probiotic microbes are components of the intestinal microbiota (also known as microflora or gut flora) in humans. Gut flora are a complex microbial ecosystem with around 101412 bacteria per millilitre, the density of bacteria in the large intestine is the highest. This is where the microbes colonise the surface of the gut and form a microbial barrier which, as and exposed barrier, selectively shields the inner body from the outside world (gut lumen). Further functions of intestinal microbiome include:
- Intestinal epithelium barrier. Intestinal epithelium cells form a mechanical barrier between the gut lumen and the inner body. The individual epithelium cells are bound together via special protein complexes known as tight junctions, which cover the entire small and large intestine and thus impede the penetration of pathogens (germs and toxins) into the body's interior.
- Gut-associated immune system. The gut is an important immune organ; around 80 % of the B cells and more than 60 % of the T cells in the body are located in the intestinal mucosa. Specialised gut epithelium cells also exist (“M cells”) which detect antigens from the gut lumen and present them to the lyphocytes found in the mucosa organised in the form of lymph follicles.
- Metabolic function. Intestinal germs convert indigestible foods parts into soluble, usable substances (e.g. polysaccharides into monosaccharides) and breakdown pathogenic micro-organism toxins. Alongside this, certain germs synthesise a range of bioactive substances, including vitamins such as biotin and vitamin K.
It's via this mechanism that the intestinal microbiome influences the function of the gut and furthermore unfolds the systemic effects on the entire body.
Useful information.
- The biological effects of the intestinal germs vary from genus to genus and are generally specific to the strain. When using probiotics, multispecies products are recommended. This allows the individual strains to complement their properties.
Information on production technology
Qualitative requirements
The company Intercell Pharma has been developing and marketing probiotic micro-organisms since 1998 and therefore has exceptional specialist knowledge in this field. Our intensive research and many years of experience has allowed our probiotic preparations to exhibit several innovative, superior properties and to fulfil all the qualitative criteria to which modern probiotics are subject.
Selecting suitable strains
The collective term of “probiotics cultures” includes bacteria genera (e.g. bifidobacteria), bacteria species (e.g. bifidobacterium longum) and bacteria strains (e.g. Bifidobacterium longum IN-7) if probiotic effects have been proven for them. Effects of probiotics always come from the individual properties which a certain bacteria strain possesses which are not automatically expected from other strains. The key qualitative criteria which probiotic strains are subject to is proof of their ability to survive in the gastrointestinal tract as well as in the end product.
Natural resistance of Intercell strains towards gastric and bile secretions
Apart from a few exceptions, probiotic strains are extremely sensitive to gastric and bile secretions and lose their viability or their activity as soon as they encounter these influences. They are subsequently only able to display a limited probiotic effect. Strains with a natural resistance survive the stomach passage unharmed and are characterised by a high level of robustness in the gut. They establish themselves more permanently onto the gut wall and are able to better suppress the unfavourable micro-organisms. To that effect, all strains are therefore tested by Intercell before use as to whether they remain stable in the gastric juices over a period of three hours without any noteworthy drop in bacteria count.
Each individual strain yields the following evidence:
- No noteworthy drop in bacteria count after a 3-hour incubation at 37° C in a gastric juice solution with pH 3.0 (corresponds to the pH value in the contents of the gut after a light meal)
- High reproductive rates also under the addition of bile in a concentration of 10 %
This allows particularly resistant, effective probiotic cultures to be used. An example of careful checking and selection is the chosen Lactobacillus acidophilus strain which, of more than 90 studied strains, is the only one to meet the conditions mentioned. Another advantage of the resistance to the natural gastric juices is the opportunity to avoid unnecessarily burdening the body through the use of galenic auxiliary and additive ingredients in the end product which are otherwise added to protect against sensitive cultures, which are not resistant to the gastric juices.
Survival in the end product/microencapsulation
Probiotic cultures can only be stored under certain conditions and lose their viability as metabolic organisms within a few days when they come into contact with water (e.g. in milk products). To make the cultures last for a longer period of time, the water is removed from the cells using a freeze-drying process until their metabolic activity is virtually brought to a standstill. As soon as the cells come back into contact with water, their metabolic process is set in motion once more and they reconstitute themselves into biologically active organisms, which are able to multiply. Nevertheless, even freeze-dried cultures gradually lose their viability within a few weeks or months (depending on the properties of the strain, storage conditions and environmental factors). In order to guarantee the high bacterial count in the end product over the total shelf life, the bacteria strains are stabilised by Intercell using two different measures.
Microencapsulation
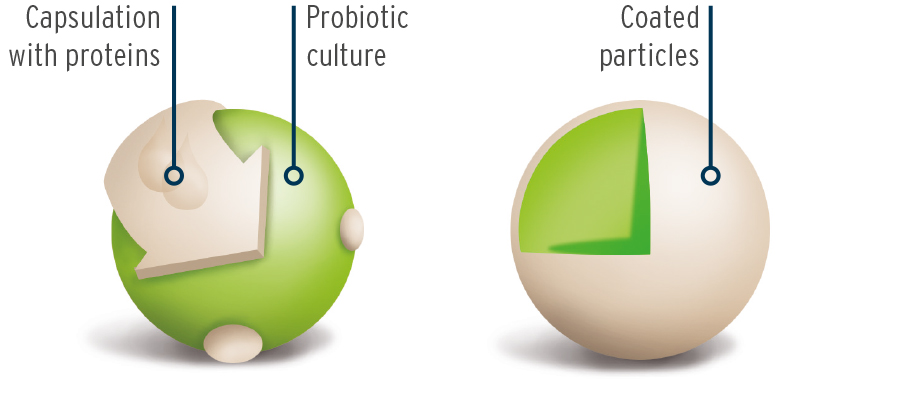 The cultures are firstly microencapsulated using an innovative process. The microencapsulation gives stability to the cell structure of the bacteria to enable the sensitive bacteria to survive better the manufacturing process and to be better preserved in higher concentrations in the end product. During the subsequent storage period, they are also better protected against outside influences and are more durable.
The cultures are firstly microencapsulated using an innovative process. The microencapsulation gives stability to the cell structure of the bacteria to enable the sensitive bacteria to survive better the manufacturing process and to be better preserved in higher concentrations in the end product. During the subsequent storage period, they are also better protected against outside influences and are more durable.
Storage stability
After production of the capsules, the second measure is safeguarding the storage stability by selecting optimal packaging materials. The objective is to have the best possible protection against oxygen and water vapour. This is why only the highest quality and best suited materials are used for packaging.
Blister packs
 The storage stability of oxygen and water vapour sensitive probiotic cultures is additionally safeguarded with the selection of optimal packaging materials. All Intercell capsules with probiotics are packaged in special blister packs which long-term packaging tests prove to be the best protection against damaging oxygen and water vapour, exhibiting the highest barrier of all packaging materials.
The storage stability of oxygen and water vapour sensitive probiotic cultures is additionally safeguarded with the selection of optimal packaging materials. All Intercell capsules with probiotics are packaged in special blister packs which long-term packaging tests prove to be the best protection against damaging oxygen and water vapour, exhibiting the highest barrier of all packaging materials.
Powdered products are filled into pharmaceutically suitable containers and induction sealed. Only induction sealing leads to a hermetically sealed closure of containers by melting together an inner seal with the edge of the container using a wax layer.
The use of this high-cost process principally enables the storage of the preparations at room temperature which really facilitates the use e. g. when travelling.
MVTR Test
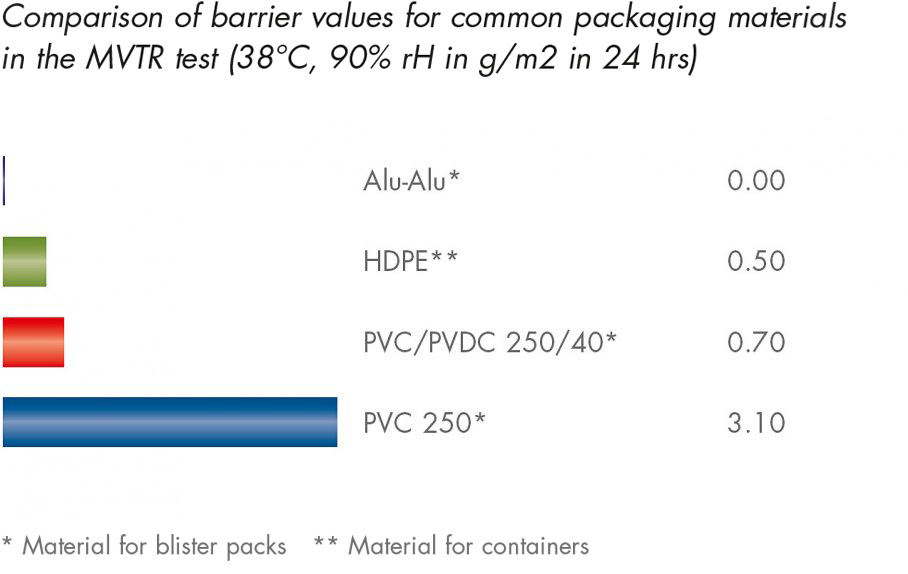 The MVTR test (Moisture Vapour Transmission Rate) provides the quantity of moisture which can be channelled through a membrane per unit of time as water vapour. The lower the MVTR value is, the more effectively the accumulation of moisture can be prevented.
The MVTR test (Moisture Vapour Transmission Rate) provides the quantity of moisture which can be channelled through a membrane per unit of time as water vapour. The lower the MVTR value is, the more effectively the accumulation of moisture can be prevented.
As can be seen from the diagram, the aluminium-aluminium blister packs offer the best barrier protection since not water vapour can penetrate the aluminium layer incorporated into the composite foil.
Packaging in containers
Food and pharmaceutical grade HDPE containers offer good barrier properties against water vapour and oxygen and, unlike glass containers, can be induction sealed. The closure represents the main weak point of containers for the penetration of oxygen and water vapour into the container. Only induction sealing by melting an inner seal with the rim of the container leads to a hermetic seal of the container's closure.
An induction sealed container can be identified by a fixed seal sitting on the opening which is hard to rip off. Induction seals differ from cheaper self-adhesive seals which are also sticky along the rim but are penetrable. They are generally easy to peel off.
Safety, product compatibility
Since probiotic preparations introduce micro-organisms into the body, the choice of cultures and the processing of products are subject to particular safety requirements.
As an essential safety requirement, all Intercell bacteria strains fulfil the precise taxonomic classification of micro-organisms to safety level 1 (no risk) and are genotyped according to species and fully identified by sequencing the 16S-rDNA ranges. In addition, they meet the internationally recognised GRAS standard (Generally Recognised As Safe) so that they are safe and suitable for consumption by humans.
The manufacturing is carried out in accordance with the strict GMP (Good Manufacturing Practice) regulations. For example, to eliminate cross-contamination or impurities during the manufacturing process, and to safeguard the high bacteria count, the eight manufacturing steps are accompanied by 17 different quality assurance measures, which are carried out a total of 61 times. This results in exceptionally high production safety and consistent quality.
Overview
Intercell Pharma probiotics exhibit the following advantages
- Natural stability of the bacteria in gastric and bile secretions
- Selection of 11 different probiotic strains for combining with small intestine specific lactobacilli and large intestine bifidobacteria
- High level of storage stability using microcapsulation
- Practicable high bacteria concentration in the end product
- The use of optimal packaging materials through high-barrier protective blister packs and induction sealed containers for maximum barrier protection
- Avoidance of gluten, lactose, artificial colour or preservatives, milk protein, sweeteners or excipients which are resistant to gastric juices.
© Intercell Pharma GmbH



